-
Credits
- Story by:
- Laura Kane & Aleksandra Sagan
- Edited by:
- Sunny Freeman & Kevin Ward
- Layout & Graphics by:
- Lucas Timmons
- Produced by:
- Megan Leach
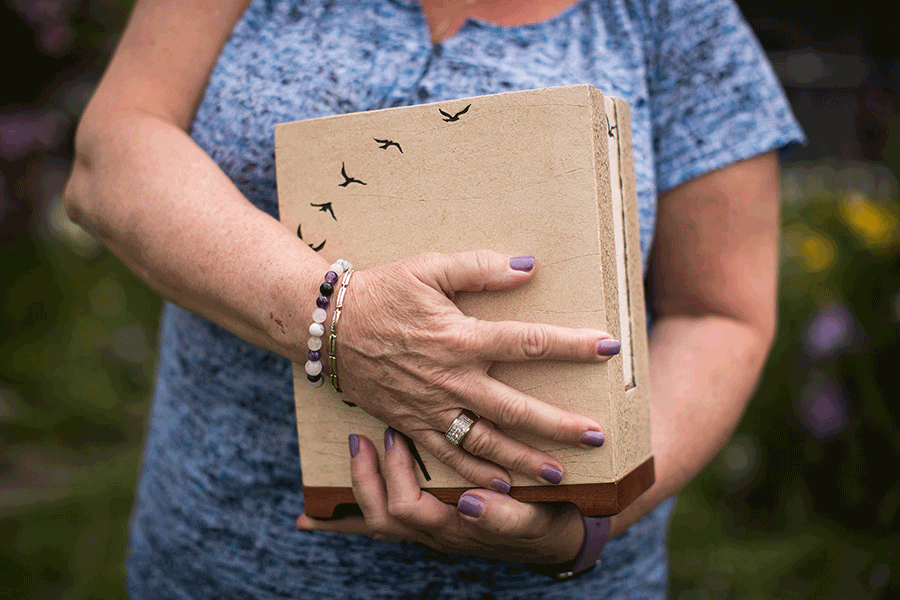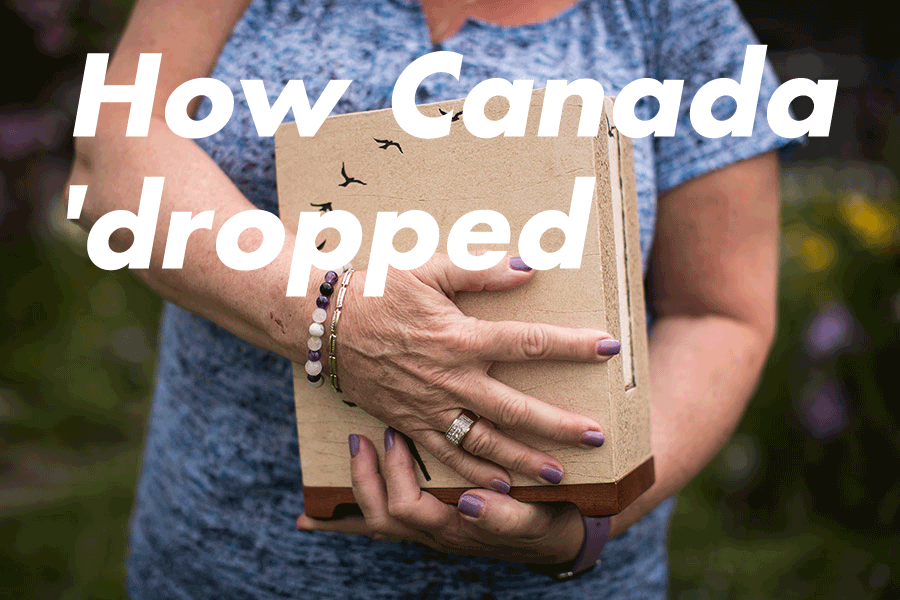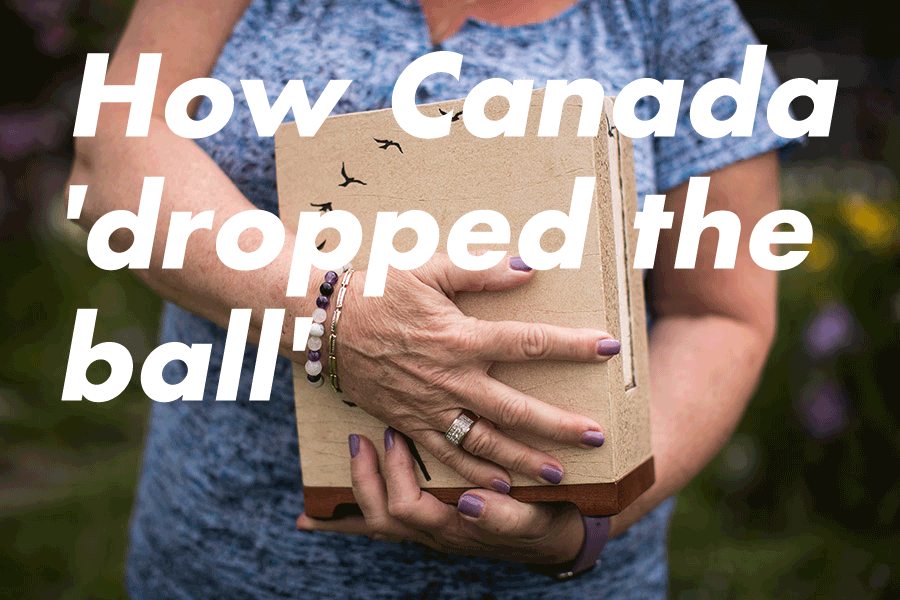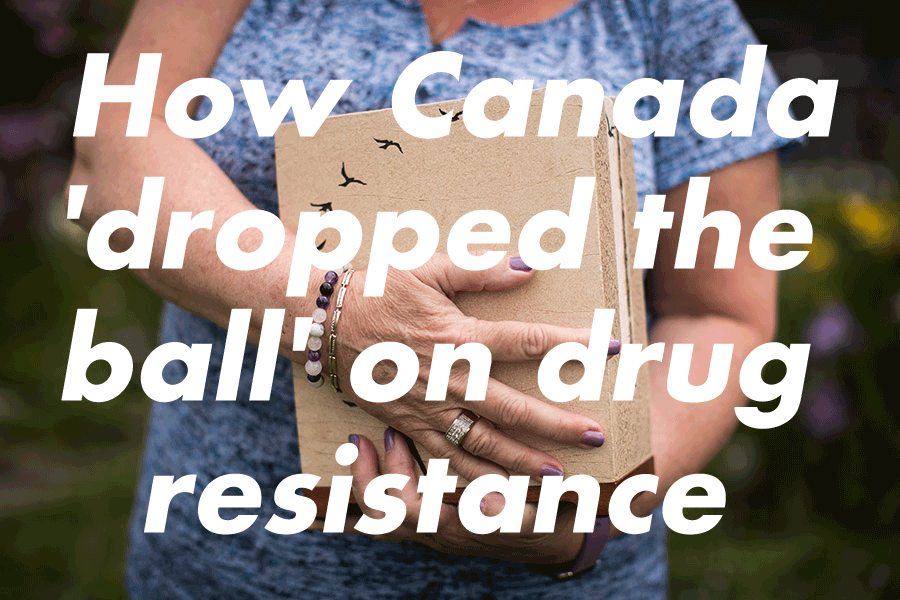
ALDERGROVE, BC — Wendy and George Gould were supposed to grow old together.
The couple found each other on a dating website when they were in their 40s. To Wendy's delight, George met all her criteria: he was kind, funny, and most importantly, he still believed in Santa Claus. They were goofy best friends who didn't want to grow up, but if they had to, they'd do it side by side.
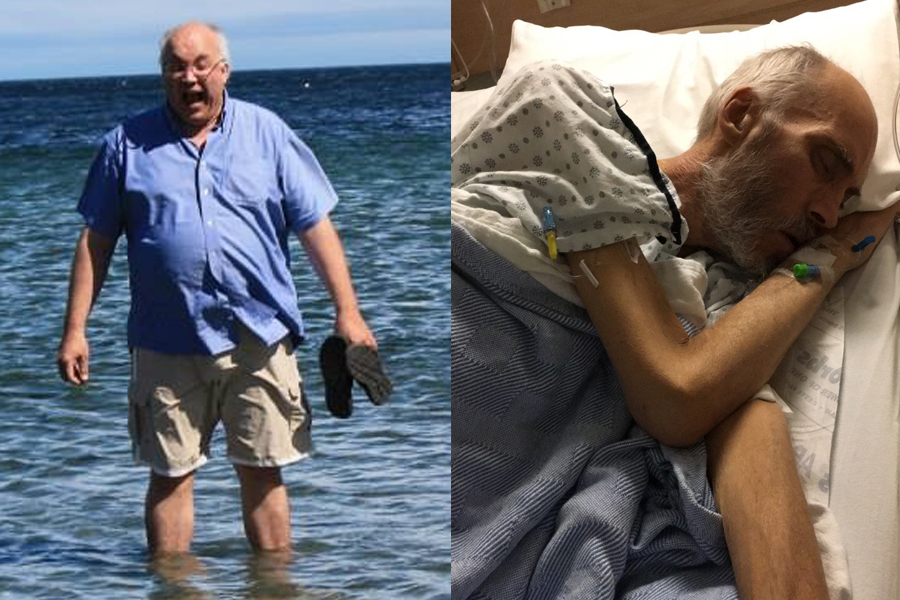
But Wendy says that future was ripped away from them when George contracted a drug-resistant superbug at a Vancouver hospital in 2016. He had undergone two surgeries to treat his stage-four colorectal cancer and was supposed to resume chemotherapy, but his wife says the infection ravaged his already fragile body and left him too weak to continue treatment.
During the final 18 months of his life, he was admitted to hospital 22 times for intravenous antibiotics that triggered violent nausea and, on some occasions, frightening hallucinations, she says. He became so thin that his skin looked stretched over his bones, and she says the 58-year-old father died in an isolation unit.
"He was supposed to be with me forever," Wendy says, wiping away tears as she sits outside the mobile home she shared with her husband in Aldergrove, B.C.
"They took his life, and they really took mine because I'm alone."
Antimicrobial resistance, or the growth of micro-organisms that fight off the drugs used to treat them, has been rising in Canada and globally for decades.
The unfettered use of antibiotics in humans and animals, coupled with environmental contamination, has helped create superbugs and made common diseases more difficult to cure. A 2016 review of antimicrobial resistance estimated 700,000 people die annually from drug-resistant infections, but the author has since updated that figure to 1.5 million.
Unless urgent action is taken, experts warn that by 2050, the annual death toll will soar to 10 million worldwide — dwarfing cancer — and drug resistance could cost the global economy US$100 trillion overall. Developing countries will experience the worst impacts, but countries like Canada are not immune: widespread international travel and trade help bacteria spread across borders.
Canada has been slow to act.
Infectious disease physicians sounded the alarm about antimicrobial resistance 20 years ago, but the federal government, provinces and territories continue to debate how to respond. Research dollars are sparse, surveillance is severely limited and the number of Canadians who die from drug resistance is a glaring unknown.
Antimicrobial resistance occurs because micro-organisms, including bacteria, viruses and fungi, evolve to resist the drugs that would otherwise kill them. Unnecessary antimicrobial use exacerbates the problem.
Antibiotics kill bacteria sensitive to the drug as well as good bacteria, leaving the cells that are drug-resistant to multiply and take over. Some bacteria can also mutate to become resistant and transfer their resistance to one another.
The swirling mass below is bacteria. The red cells are drug-resistant. The blue cells are bacteria that are sensitive to drugs.
In a large population of bacteria, a few cells are drug-resistant.
Antibiotics kill the sensitive bacteria, leaving the resistant population behind.
The resistant bacteria multiply and replace the cells that were killed off.
Bacteria can also transfer their resistance to one another.
NextDrug resistance was a growing concern in 1997 when Dr. John Conly, then-president of the Canadian Infectious Disease Society, decided to act. He worked with Health Canada to organize a conference in Montreal that generated 27 recommendations and the beginnings of a national committee to co-ordinate a response.
Former prime minister Jean Chretien's government granted $300,000 in annual funds to the Canadian Committee on Antibiotic Resistance. It published a proposed national plan in 2004 that recommended actions to improve surveillance and reduce unnecessary antibiotic use.
"And then it just sat on a shelf," recalls Conly, former chairman of the committee.
"The federal government at the time, they seemed to lose interest. There was a lot of downsizing."
The committee lost funding in 2008. Its final report suggested it never received sufficient funding or adequate staffing to co-ordinate drug resistance activities nationally and called for a more comprehensive approach.
The Public Health Agency of Canada took the lead on antimicrobial resistance. But in 2015, the federal auditor general found the agency and Health Canada had not fulfilled key responsibilities to mitigate the public health risks posed by the issue.
The agency had talked with provinces and territories over five years, but failed to achieve a consensus on the scope of a plan, the auditor general concluded.
"The ball was dropped for many years," says Conly.
"It's all too easy to say, 'Well, there were other headlines and other fires to put out.' But it's not all about crisis of the day."
He likens antimicrobial resistance to climate change: "a slow-moving tsunami."
Even as patients' lives rapidly deteriorate, the federal government's approach to drug resistance over the past decade has been something of a bureaucratic odyssey.
The government produced a federal framework in 2014. The next year, it released a federal action plan that committed $20 million to a research project and established a new body to integrate existing surveillance.
But the plan was limited to the federal government and one that lays out responsibilities for the provinces and territories has not been produced. The federal government has been consulting since 2011, but only issued its framework last September. It hopes to publish the plan next year.
The framework, which describes general ambitions for surveillance, stewardship, innovation and infection control, is a milestone, says Dr. Howard Njoo, Canada's deputy chief public health officer.
"Glacial."
The government's pace on antimicrobial resistance according to Dr. Andrew Morris
He can understand why some doctors have been frustrated with the pace of change, but insists there has been movement over the years.
"It's hard, maybe, to appreciate from the outside all of the work that happened in the preceding years," he says.
But Dr. Andrew Morris, director of the antimicrobial stewardship program at Sinai Health System/University Health Network, sums up the government's pace on antimicrobial resistance using another word: "glacial."
Part of the problem, he says, is the government hasn't spent enough money.
The public health agency spent $6.9 million on programs related to antimicrobial resistance in 2016-17, about 1.2 per cent of its $589 million budget. The Canadian Institute for Health Research spent just $280 million on the issue over 17 years — a little more than it spends annually on cancer and oncology.
The federal government, meanwhile, has no grasp of how many Canadians are dying in hospitals from drug-resistant infections and statistics are not readily available.
The national surveillance system monitors deaths in 60 large hospitals from four kinds of superbugs, for which it provides mortality rates of between three and 40 per cent. Doctors might attribute a patient's death to their underlying illness rather than the infection, potentially obscuring the impact of drug resistance in Canada.
The Canadian Institute for Health Information told The Canadian Press it would cost more than $12,000 and require two weeks of staff time to quantify the number of death certificates that list such infections, before saying it could not be done at all. Statistics Canada provided a roughly $25,000 price tag and a timeline of nearly three months. The agency is working on a scaled-down version of the request, but won’t be able to provide data until mid-July.
The death certificate Wendy Gould has for her husband doesn't list a cause of death, which is standard for certificates issued to relatives in B.C. But she believes the drug-resistant infection killed him because it prevented him from continuing his cancer treatment, and she wants the hospital to take responsibility.
She provided a letter to The Canadian Press on health authority letterhead and sent to George in March 2017. It informs him that he was one of three patients who contracted New Delhi metallo Escherichia coli. All three received care at the hospital's endoscopy clinic in July or August 2016 and while the source of the infection is unknown, there is a "possibility" it was connected to an endoscope used in his procedure, the letter says.
Wendy filed a lawsuit in the Supreme Court of British Columbia against Vancouver Coastal Health, the health authority that operates Vancouver General Hospital, alleging the superbug ultimately led to George's death. Her claim has not been proven in court.
When asked about the letter, the health authority referred to its statement of defence filed in court. The statement does not address the letter, but it confirms George had New Delhi metallo Escherichia Coli. The health authority, however, denies knowledge of the infection's source or that it caused or aggravated any of his medical issues or contributed to his death. The authority maintains it tested the endoscopy clinic after George contracted the superbug and found no organisms of the type he contracted.
Newborns, people with weakened immune systems and the elderly are especially susceptible to drug-resistant infections. Cystic fibrosis patients are particularly prone due to their chronic lung infections, which are often treated with antibiotics. Over time, the bacteria in the lungs can become resistant.
Marika Archambault-Wallenburg grew up with cystic fibrosis, but never had a superbug before she was hospitalized at the age of 26. Over the following year and a half, she battled no less than four drug-resistant infections in Canadian hospitals, her father, John Wallenburg, contends.
"Hospitals are dangerous places. They're dangerous places in general, but for people who are immunocompromised, they're particularly dangerous," says Wallenburg, also chief scientific officer for Cystic Fibrosis Canada.
The infections sapped Marika's morale at times, but not her hope.
She spent her days in hospital scrawling dreams on homemade paper about life with new lungs, musing about a motorcycle trip to Yukon.

Marika did not survive long enough to receive the transplant. Her health rapidly deteriorated and a week before her 28th birthday, she died in hospital due to complications from her cystic fibrosis.
"She had friends who said she was fearless. It really wasn't that she was fearless," says her father.
"She placed her fears on a different scale than most of us. Because the fears she faced really were a hell of a lot more significant than what others fear."
Infectious disease physicians are seeing more cases like Archambault-Wallenburg's. But Canada still doesn't have a strong understanding of where resistance is developing or how antibiotics are being used, says Morris of Sinai Health System/University Health Network.
The country has two monitoring programs in place. One collects data from hospitals, while the other gathers information about antimicrobial use in animals from farms raising pigs, chickens and turkeys, as well as resistance along the food chain.
Data from both programs — as well as some information from provincial labs and other sources — feeds into annual reports published by the Canadian Antimicrobial Resistance Surveillance System. The system also buys data from a company called IQVIA on prescriptions written by doctors and dispensed by pharmacies, as well as antimicrobials purchased by hospitals.
But Morris calls the surveillance system a "well-intentioned piece of crap."
"Imagine, our federal government, on something so important as health care, is paying a proprietary company to understand how antibiotics are used throughout the country," he muses.
"I don't believe any of that (IQVIA) data. For antibiotic use, I think it's massively wrong. No one has ever validated it."
IQVIA spokeswoman Madeline Gareau says clients get full access to the data they purchase as well as insight into how it's compiled if they choose. It's misleading to say the Canadian government cannot validate the data, she says, adding that IQVIA does not conduct any interpretive work for the surveillance system so it cannot comment on conclusions drawn in its reports.
The Public Health Agency of Canada maintains that while it's true that the data cannot be formally audited, some independent verification does take place.
"(A) well-intentioned piece of crap."
the Canadian Antimicrobial Resistance Surveillance System Dr. Andrew Morris
"We are certainly exploring other sources of data. Right now, it's something we've got that we're trying to make the best use of," says Dr. Chris Archibald, the agency's surveillance and epidemiology director.
He acknowledges there are gaps in the surveillance system, including that it does not adequately measure resistance in the community or in small, rural and northern hospitals. The agency is looking at expanding its coverage and has done some short-term studies of such facilities.
The program that monitors antimicrobial use in animals is also missing data, including a lack of oversight of use in beef cattle.
But consultations are set to begin on how to collect such information, says Dr. Rebecca Irwin, a veterinary epidemiologist who oversees the program.
"We recognize it as a gap. We'd like to have all our commodities represented."
Provinces also have surveillance systems, but their methods are so variable that the statistics can't be aggregated. For example, B.C. and Ontario collect data on several superbugs, while Saskatchewan only monitors C. difficile.
The superbug that Wendy Gould blames for her husband's death was an E. coli strain containing New Delhi metallo-beta-lactamase (NDM), an enzyme resistant to crucial antibiotics called carbapenems. The enzyme was first detected in 2008 in a Swedish patient who had received treatment in an Indian hospital. Within two years, NDM reached North American shores.
Dr. Linda Hoang, a medical microbiologist with the British Columbia Centre for Disease Control, was so alarmed by the rise of NDM and other organisms that can resist carbapenems that she publicly called for a provincial surveillance system in 2014.
"We're seeing emergence of a highly resistant organism that could potentially take over our health-care system and make it very difficult to treat patients," she says. "If we don't really know what the burden of disease is, we can't respond to it."
Following Hoang's call, B.C. launched a provincial network to measure several superbugs, including those resistant to carbapenems, in all 80 of its acute care hospitals. She is now co-medical director of the network.
The latest report shows 86 new cases of carbapenem-producing organisms (CPO) in 2016-17, including 59 cases of NDM. Nearly seven-in-ten patients with CPOs had accessed health care outside of Canada in the previous year. Of the remaining cases, roughly half were likely acquired in B.C. hospitals, while the other half are a mystery, either emerging in the community or through some unidentified mechanism, Hoang says.
"If we don't really know what the burden of disease is, we can't respond to it."
British Columbia Centre for Disease Control medical microbiologist Dr. Linda Hoang
She's encouraged that most are travel-related because that's how the organisms reach the Western world in the first place and suggests there is not widespread transmission in B.C. Still, she calls for a global effort to support countries where drug resistance is more endemic.
"It's not only South Asia or southeast Asia that we're seeing resistance. South America, Central America, Europe, U.S. — it's everywhere," she says.
"There's only so much we can do in holding up our gate. We need to support ... the endemic countries to minimize their numbers and their risk."
The Canadian government gave $9 million last year to the World Health Organization for a project to combat antimicrobial resistance in developing countries. It also contributes to international funding organizations in resistance-plagued countries, including India and South Africa.
While Wendy Gould turned to the courts for answers after her husband's death, Vanessa Carter in Johannesburg, South Africa, uses social media to spread awareness about the prevalence of superbugs so patients can advocate for themselves.
South Africa might seem distant, but Canada donates to the country because drug-resistant bacteria will inevitably travel across borders and infect Canadian patients. Some 22,000 South Africans visited Canada last year, while Canadians visited South Africa 167,000 times.
Carter was in a car crash when she was 25 that obliterated the right side of her face, smashing every facial bone and destroying her eye. She underwent numerous surgeries, including the insertion of a 3D-plastic implant above her cheekbone. A month after the operation, she noticed pus seeping from the prosthetic.
Doctors said she needed a procedure to remove the infected tissue and a course of antibiotics. The infection consistently reappeared even worse, despite repeating the treatment multiple times. Finally, a surgeon insisted she take out the implant. After its removal, she learned her infection had been drug-resistant.
Carter had never heard of her diagnosis: methicillin-resistant staphylococcus aureus, or MRSA. But as she read more about it, she grew furious.
She could have unknowingly transmitted the superbug to her children at any time and it could have spread to her bloodstream and killed her.
"It had been such a traumatic experience, that 11 months of waking up every day, cleaning the infection and saying, 'Is this going to get worse tomorrow?' And not having any control of what was going on," she recalls.
Carter's face was eventually restored through a combination of surgeries, some 10 years after her car accident.
She hadn't taken a photo of herself in a decade and read an article titled "Selfies for Dummies" before posting her first one to Facebook in 2013.
"It was an amazing moment," she says. "I made this whole album of selfies because I felt so good."
Carter, 39, now uses social media and her website to advocate on behalf of patients, who she says need to be empowered with information so they know when to say no to unnecessary courses of antibiotics.
"If I had been more informed ... I could have put my hand up and said, 'I know that antibiotics can cause resistance. Please, can I go for a test?'"
"One of the missing pieces of the puzzle is the patient."